New York, New York--(Newsfile Corp. - January 7, 2026) - Frost & Sullivan has released a white paper titled, "How Leading Global Pharma Embraces AI to Automate Regulatory and Medical Documents with Quality Control" (hereinafter referred to as the "White Paper"). As artificial intelligence becomes central to regulatory and medical writing, the pharmaceutical industry is undergoing rapid transformation. Organizations face mounting documentation demands, cost pressures, and the need to bring therapies to patients faster. Real-world pilots and production deployments show that generative AI can significantly improve authoring speed, scalability, and consistency-while elevating medical writers' roles and reducing operational burden.
Drawing on perspectives shared in public forums at AMWA 2025, a DIA webinar in 2025, and an EMWA virtual conference in 2025, this White Paper synthesizes insights from senior professionals across global biopharmaceutical companies, regulatory agencies, and leading technology providers. It distills foundational strategies behind successful AI transformation and provides a roadmap for responsible, enterprise-scale adoption in regulatory and medical writing.
1. The Regulatory Writing Challenge: Why Change Is Essential
The White Paper notes that regulatory writing has become more complex, data-dense, and time-critical than ever. With drug development costs exceeding USD 2.3 billion and timelines often spanning 10-15 years, even modest delays in documentation can have major financial and patient-access consequences. Writing teams confront persistent bottlenecks: large document volumes, constantly evolving regulations, slow cross-functional review cycles, and writer burnout from repetitive drafting.
From Frost & Sullivan's perspective, these pressures make regulatory writing a prime target for transformation. To keep pace with rising scrutiny and operational demands, automation and continuous improvement have become essential.
"Regulatory writing has outpaced what manual processes can handle. Automation is essential to keep up, so experts can focus on higher-value work."
- Eunice Youhanna, Industry Advisor, Health & Life Sciences, Microsoft
2. Regulators Focus on Quality, Not Tools
The White Paper highlights that regulators are not focused on how documents are drafted internally, but on the quality of what is ultimately submitted.
Regulatory experts emphasize that while the FDA does not regulate the writing process itself, submission documents must remain clear, accurate, and reliable. Human expertise therefore remains essential: AI may assist with drafting and structuring, but qualified professionals must validate all content. In Frost & Sullivan's analysis, regulatory expectations consistently center on three principles:
Accountability - Human review and responsibility for content
Traceability -linkage of AI-generated content to underlying source data and evidence
Quality parity - AI-assisted documents must meet the same scientific and regulatory standards as human-authored documents
AI is acceptable when used within a transparent, well-controlled, human-governed framework in which quality and traceability are demonstrably maintained.
"While FDA does not regulate the internal medical writing process, the agency has a strong interest in ensuring the quality and accuracy of regulatory submissions, regardless of how they are generated."
- John Jenkins, MD, Former Director, Office of New Drugs, FDA
"AI is exceptionally well suited for reporting and summarizing clinical results, but interpretation, medical judgment, and risk evaluation must remain human-driven."
- Ellis F. Unger, MD, Principal Drug Regulatory Expert, Hyman, Phelps & McNamara; Former FDA Office Director
3. How Leading Companies Started Their AI Journey
According to the White Paper, early adopters in global pharma rolled out GenAI through focused, phased programs, targeting high-impact, low-risk document types with structured, repeatable patterns so they could demonstrate value quickly under close human oversight.
"If you bring a blockbuster drug to market just one month faster, that can represent over $400 million in additional revenue. That's why AI-driven acceleration is no longer optional for pharma."
- Eunice Youhanna, Industry Advisor, Health & Life Sciences, Microsoft
3.1 Securing Executive Sponsorship
Executive sponsorship consistently emerges as a critical success factor: senior leaders set the vision, align teams on strategic priorities, focus investments, and clear governance roadblocks so pilots can scale.
"Best practice is aligning on the long-term goal, defining mid-term value, and assessing short-term gains. When all executive stakeholders share this understanding, AI becomes a truly transformative force."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
3.2 The "High Volume, Low Risk" Pilot Strategy
The White Paper highlights that most global pharma organizations began with Clinical Study Reports (CSRs) and patient narratives-high-volume, structured, writer-owned deliverables-using these "high volume, low risk" pilots to compare AI with legacy processes, prove quality, and build trust before expanding.
"The key was really to start with high-volume, low-risk use cases, which allowed us to build trust and transparency within the organization and validation, and then really scale that based on the measurable impact we're seeing."
- Senior analytics leader at a global biopharmaceutical company
3.3 Building Internal Consensus
To move beyond technology demos, leading companies engaged medical writers, clinicians, statisticians, regulatory and quality experts, and IT early, co-creating AI workflows that reduced resistance, created internal champions, and accelerated deployment.
"Once we had the relevant stakeholders internally aligned… it became a straightforward and quick process. We needed data scientists, IT partners, clinicians, stats programming - to make sure we had the right experts at the table."
- Senior regulatory medical writing leader at a global biopharmaceutical company
4. Implementation Best Practices: Lessons from Successful Rollouts
The White Paper reports that successful AI transformation requires strong, visible executive leadership: in leading global pharmaceutical organizations, committed sponsors articulate the strategic value of AI, unlock resources, clear organizational roadblocks, set realistic but ambitious expectations, and use early wins and lessons learned to build company-wide momentum.
4.1 Key Strategies for Enterprise-Grade AI Adoption
Frost & Sullivan summarizes a consistent set of strategies when operationalizing AI for regulatory and medical writing for leading global pharma companies: they align adoption with purpose such as reducing burnout, elevating job satisfaction, and connecting work to patient and scientific impact, engage cross-functional stakeholders early and often, co-create and iterate with clinicians, writers, and operations to build trust, share transparent benchmarks and learning, and invest in training, internal "champions," and hands-on enablement for users at all levels.
"There's no point finding a single-point solution that only solves one document type… We needed something fit for purpose, scalable across the organization."
- Senior regulatory medical writing leader at a global biopharmaceutical company
4.2 Change Management and People Transformation
The White Paper underscores that, across global pharma, the biggest barrier to AI adoption is cultural rather than technical. Success hinges on framing AI as augmentation-not automation-supported by targeted training, clear communication, visible early champions, and open discussion of concerns. Done well, AI frees scientific and regulatory experts to focus on higher-value work-strategic thinking, interpretation, and collaboration-while improving consistency and overall document quality.
"Successful AI adoption isn't a technology challenge per se. It's really a people and process transformation. A real best practice was framing AI as augmentation, not automation. That mindset shift is helping teams see AI as a strategic partner, not a threat."
- Senior analytics leader at a global biopharmaceutical company
4.3 Vendor and Solution Criteria
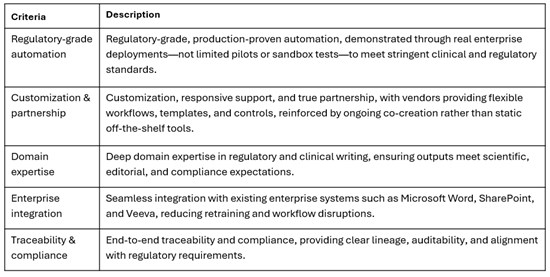
When evaluating AI solutions for regulatory and medical writing, global pharmaceutical companies apply a rigorous framework to ensure platforms are reliable, compliant, scalable, and suited to complex regulated workflows.
Figure: Core Selection Criteria
By prioritizing these elements, global pharma ensures AI solutions are enterprise-ready and built for long-term success.
"They really were dedicating themselves to regulatory and clinical medical writing, not AI in general… and they gave us a package that really - as you said - agency acceptance, going through all the testing for QA, IT QA, and all of your return on investment."
- Karen J. Devcich, Former Vice President, Medical Writing, Quality & Editing, ICON plc
4.4 Building a Phased Adoption Roadmap
The White Paper notes that, across global pharmaceutical organizations, a phased, multi-horizon roadmap has become the leading practice for scaling AI-aligning long-term ambition with near-term results, demonstrating early value, and keeping executives, operations, and technology partners aligned as adoption expands.
Figure: Three-Horizon Adoption Framework
"Align on the long-term goal, define the midterm value, and assess the short-term gain. The visionary goal aligns with executive stakeholders-understanding AI is going to make a revolutionary change, but we just have to start and kick off the process."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
4.5 Implementing a Robust Quality Control Framework
In regulated environments, the White Paper stresses that the risk of AI "hallucinations" makes accuracy, traceability, and scientific rigor essential. Leading pharmaceutical organizations address this through structured, multi-layer safeguards so that AI strengthens-rather than compromises-regulatory submissions.
Multi-Layer Quality Assurance Framework:
- Clear accuracy criteria: Defined metrics to distinguish factual accuracy from interpretation.
- Source-grounded generation: Outputs anchored in validated source documents to avoid unsupported content.
- Automated benchmarking: Comparison with gold-standard documents, with targeted human review for complex sections.
- Expert oversight: Medical writers, clinicians, statisticians, and regulatory experts validate accuracy and compliance.
- Continuous improvement: Feedback loops and performance metrics drive ongoing refinement.
By combining structured controls with human oversight, organizations make AI a driver of higher accuracy and operational confidence-not an added risk.
"From FDA's standpoint, quality is the main concern. Ideally, reviewers should not even know whether a document was or wasn't generated with AI - the quality should be high and consistent."
- John Jenkins, MD, Former Director, Office of New Drugs, FDA; Former Principal, Drug & Biological Products, Greenleaf Health
"Quality measurement, quality management, quality assurance, and quality improvement are critical. AI can flag discrepancies that humans might overlook."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
"As a medical writer… instead of re-typing and re-checking tables over and over, this tool catches inconsistencies and lets me focus on real scientific thinking."
- Karen J. Devcich, Former Vice President, Medical Writing, Quality & Editing, ICON plc
5. AI-Powered Medical Authoring Tools
The White Paper finds that, as regulatory and medical writing grows more data-dense and time-critical, AI-powered authoring tools are moving from pilots to enterprise production. Across large pharmaceutical organizations and CROs, early adopters report that these platforms automate data-intensive groundwork, enhance traceability across document families, improve consistency at scale, and deliver 30-50% reductions in end-to-end CSR timelines-while scientific ownership remains firmly with medical and regulatory writers.
From Frost & Sullivan's perspective, this shift is being enabled primarily by specialized, regulatory-focused AI platforms rather than generic, horizontal tools. In industry discussions, solutions developed by providers such as AlphaLife Sciences were frequently cited as examples of "production-grade" GenAI for regulatory and medical writing-capturing lessons from early adopters and making them accessible to a broader set of sponsors
5.1 Human-Centered Design for Regulated Workflows
Medical writers operate in tightly governed environments with templates, controlled vocabularies, CDISC and related standards, formal review processes, and strict audit expectations. Regulators care less about how drafts are technically produced than about quality, accuracy, consistency, and inspectability. As a result, the most successful AI systems are those that embed into existing workflows instead of asking writers to work in parallel tools.
Regulatory-focused AI platforms-including those developed by companies such as AlphaLife Sciences-typically follow a similar design pattern: they operate directly within Microsoft Word to support drafting, TFL summarization, and embedded quality checks; integrate with RIM and enterprise document management systems such as Veeva Vault and other Microsoft 365-based repositories; and apply governed templates, prompts, and QC rules aligned with corporate and regulatory standards.
"You can judge a company by the quality of its documents. AI can be trained to avoid the common pitfalls of regulatory writing and help produce clear, precise, and consistent submissions-at scale."
- Ellis F. Unger, MD, Principal Drug Regulatory Expert, Hyman, Phelps & McNamara; Former FDA Office Director
"This is not general-purpose AI. Quality measurement, quality management, and quality assurance must be built specifically for regulatory content-that's where real safety and trust are created."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
5.2 AI Support Across the Drug Development Lifecycle
According to the White Paper, AI now supports a broad range of regulatory and clinical document types, with greatest value in high-volume, structured, table-driven deliverables:
Clinical Study Reports (CSRs): AI ingests protocols, SAPs, and outputs to generate structured first drafts in hours rather than weeks, aligning text with tables and figures and applying automated QC.
- Clinical Summaries (CTD 2.7.3 / 2.7.4): AI consolidates TFLs, harmonizes endpoints, and creates traceable narratives across multiple studies.
- Protocols, Amendments, and Safety Reports (DSUR / PBRER): AI accelerates synopsis and template drafting, identifies impacted sections, proposes targeted updates, and helps keep safety narratives synchronized over time.
Across the portfolio, automation accelerates reporting-intensive documents while human experts retain responsibility for interpretation and judgment.
"When domain knowledge, structure, and quality control are built in, AI can reliably automate first-draft generation across the regulatory document lifecycle-at scale. When multiple document types are onboarded together, 30-50% end-to-end lifecycle time reduction is consistently achievable-not just for a single document, but across the submission workflow."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
5.3 The "In-the-Flow" Integrated Workspace
Sustained adoption depends on operational fit: AI must sit in the flow of regulated work, not in a separate tool. Leading platforms let teams work in Word while accessing RIM and document systems, triggering governed AI drafting, pulling validated references, and writing content and metadata back into secure enterprise systems to maintain a single source of truth and full auditability-turning isolated pilots into scalable, enterprise-grade medical authoring capabilities.
In Frost & Sullivan's analysis, vendors that embody this model-such as AlphaLife Sciences in the regulatory and clinical writing domain-are shaping the emerging benchmark for how AI is operationalized across biopharmaceutical organizations globally.
"With embedded quality control, AI can detect discrepancies that humans may overlook, strengthening both consistency and regulatory confidence across interconnected documents."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
"What we like about AlphaLife Sciences is that they are not a proof-of-concept startup-they are already in production across small, mid-size, and large enterprises."
- Eric Henze, Senior Health Industry Digital Strategist, Microsoft
6. Early Results: ROI and Value Realization
The White Paper shows that early deployments across large pharma and global CROs show consistent, measurable benefits from AI-assisted regulatory and medical writing, especially in speed, quality, and workforce experience, and are now being expanded to additional document types.
6.1 Acceleration in Authoring and Timelines
Organizations report first drafts produced in hours or days instead of weeks, 30-50% reductions in CSR timelines, and faster delivery to sponsors and regulators, enabling earlier review and better adherence to clinical program schedules.
"The speed of that first draft is phenomenal… and that gave us time to do the reviews and thinking and get the documents faster to our sponsors. Our sponsors were very happy."
- Karen J. Devcich, Former Vice President, Medical Writing, Quality & Editing, ICON plc
"We're seeing 30-50% reduction in draft-deliverable timelines for real customers."
- Eric Henze, Senior Health Industry Digital Strategist, Microsoft
6.2 Quality, Consistency, and Review Efficiency
According to Frost & Sullivan, speed gains come alongside quality improvements: automated checks and AI-assisted summaries reduce manual QC effort, catch discrepancies between tables, listings, and text, improve cross-document consistency and traceability, and shorten review "touch time" so teams can focus on scientific and regulatory issues rather than basic corrections.
"I would never compromise on the quality of the documents my team produces - that's simply non-negotiable. We wouldn't be going down this route if we didn't believe we could maintain that quality."
Senior regulatory medical writing leader at a global biopharmaceutical company
6.3 Impact on Medical Writer Experience and Value
The White Paper also notes that AI also changes the work experience: it reduces repetitive drafting and data-to-text work, frees writers from re-typing tables and boilerplate, and lets them focus on interpretation, messaging, collaboration, and alignment with regulatory strategy and patient impact-raising engagement and reducing burnout.
"It wasn't really about efficiency per se. It was really about freeing up the time of our experts for higher-order, strategic thinking and improving consistency across our submissions."
Senior analytics leader at a global biopharmaceutical company
"When writers see the technology level the playing field-producing high-quality drafts regardless of seniority or location-it's transformative."
- Karen J. Devcich, Former Vice President, Medical Writing, Quality & Editing, ICON plc
7. The Future: From Automation to Orchestration
7.1 Expanding Document Type Coverage
While early AI deployments focused on CSRs and safety narratives, the White Paper finds that leading organizations are now extending support across the full regulatory dossier-including clinical, CMC, and non-clinical domains-and into summary documents and clinical overviews where synthesis and cross-document reasoning are essential. AI increasingly helps maintain relational structures across documents, supporting alignment, consistency, and accuracy across entire submission packages.
"We began with CSRs, but once writers saw the gains in speed, consistency, and quality, it became clear the same capabilities could extend across many document types. The tool reduces inconsistencies, strengthens summaries, and gives writers more time to think - not just fill in tables."
- Karen J. Devcich, Former Vice President, Medical Writing, Quality & Editing, ICON plc
" Agentic AI will ultimately map the full hierarchy of information, giving us a connected content ecosystem that delivers unmatched consistency, quality, and efficiency.
- Sharon Chen, Founder & CEO, AlphaLife Sciences
7.2 From Automation to Orchestration
The industry is now moving, as Frost & Sullivan observes, from AI-assisted drafting to full workflow orchestration. Agentic AI coordinates updates, monitors dependencies, triggers actions as data change, and maintains alignment across documents-enabling faster, more reliable submissions, improved traceability, automated propagation of updates, and a greater focus from experts on strategy and science rather than mechanics.
"I definitely see the next phase moving from automation to orchestration. The mission is ultimately faster, smarter submissions, reducing duplication, improving traceability, while freeing up our experts for strategy and science-related activities."
Senior analytics leader at a global biopharmaceutical company
8. Conclusion: The Imperative for AI Adoption in Pharmaceutical Medical Writing
The White Paper concludes that AI for regulatory and medical writing has moved from promise to proven value: leading pharma already report 30-50% faster document timelines, greater cross-document consistency, and the ability to redeploy medical writers to higher-order scientific work.
Regulators are increasingly open to AI-assisted submissions when quality, accuracy, and traceability are maintained. The question is no longer whether to adopt AI, but how quickly organizations can scale it across regulatory workflows through executive sponsorship, high-value pilots, targeted training, and enterprise-level orchestration.
"Intelligence has effectively become a utility-it's now on tap. When every employee can access it, productivity, insight, and business value transform"
- Eric Henze, Senior Health Industry Digital Strategist, Microsoft
"The future of regulatory excellence will belong to organizations that operationalize AI today-not tomorrow."
- Sharon Chen, Founder & CEO, AlphaLife Sciences
About Frost & Sullivan
Frost & Sullivan, the Growth Partnership Company, works in collaboration with clients to leverage visionary innovation that addresses the global challenges and related growth opportunities that will make or break today's market participants. For more than 60 years, Frost & Sullivan has been developing growth strategies for the global 1000, emerging businesses, the public sector, and the investment community.
Media Contact
Contact: Qian Li
Company Name: Frost & Sullivan
Website: http://www.frostchina.com
Email: qian.li@frostchina.com
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/276763