Tickers: XCNQ:BLR, XOTC:BLRZF, PINX:BLRZF
Tags: #Finance
Vancouver, British Columbia - TheNewswire - April 14, 2020 - Blackhawk Growth Corp. (CNSX:BLR) (the "Corporation" or "Blackhawk"), is pleased to announce that it has entered into a letter of intent (the "Letter"), dated effective April 11, 2020, with Emergence Technology Pty. Ltd. (the "Vendor") to distribute a 2019-nCoV Ab test kit (the "Test Kit") used in the detection of COVID-19. The Letter contemplates that Blackhawk would acquire the rights to distribute Test Kits in Canada, Mexico, Germany, Switzerland and Austria (the "Acquisition Territories"), subject to the requirements of applicable medical regulations in these jurisdictions.
While the Test Kit was submitted to Health Canada for clearance on March 31, 2020, at this time distribution of the Test Kit has not been approved for use in Canada and there can be no guarantee that such approval will be granted in a timely fashion, or at all.
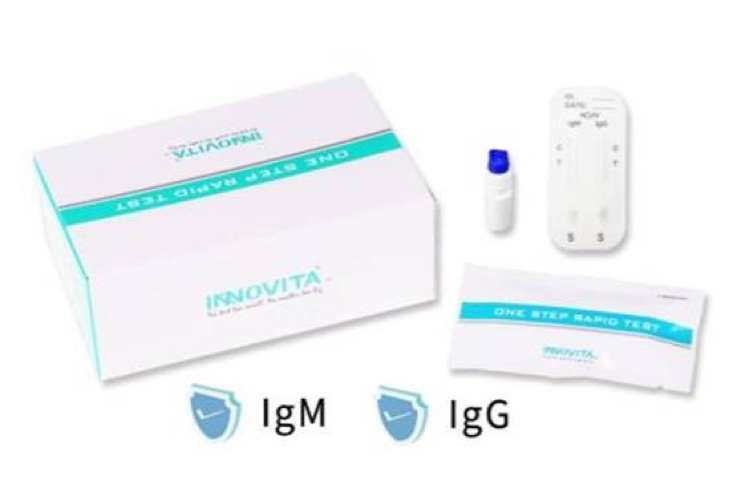
The Test Kit is a small disposable point-of-care test (POCT), that can be used in clinics, hospitals, pathology labs or in remote sites administered by healthcare professionals. The device itself is based on lateral flow colloidal gold-based detection technology that detects viral specific IgG/IgM antibodies present in a few drops of blood from a finger-prick. The device only requires 10 microlitres of patient serum or plasma, or 20 microlitres of whole blood, which is loaded on one end of the Test Kit together with a buffer mix, which then mixes with COVID-19 spike proteins (S) labelled with colloidal gold and migrates along the device to an area of immobilized antibodies that captures COVID-19 specific antibodies. If virus specific IgG or IgM antibodies from the patient are present, compounds are formed, which show up as a distinctive purple band on the strip. The results are obtained within 3 to 15 minutes, and do not require specialised laboratory equipment such as those that use real-time RT-PCR (reverse transcriptase-polymerase chain reaction) technology.
Picture of Innovita's COVID-19 Test above
The Test Kit is developed and manufactured by Innovita (Tangshan) Biological Technology Co., Ltd. ("Innovita") in China. Established in 2006 in Beijing, Innovita is a leading manufacturer of diagnostic solutions for respiratory pathogens diagnosis, striving for a more efficient healthcare system to enhance the health and well-being of everyone in the world. Innovita is currently in the process of manufacturing and distributing the Test Kits in China. Readers are encouraged to visit their website for further information regarding Innovita (http://www.innovita.com.cn).
The Vendor has an existing distribution relationship with the Innovita which permits it to market and distribute the Test Kits in a number of jurisdictions, including the Acquisition Territories. The Test Kits are already fully approved and have a CE mark in Europe as well as by the respective health authorities in China, the Philippines and Australia among other jurisdictions.
As global cases exceed 1,900,000 and continue to rise, Dr Tedros Adhanom Ghebreyesus, director-General of the World Health Organisation emphasized the critical need to escalate testing, isolation and contact tracing efforts, which he termed the "backbone" of the response. He further emphasised "We have a simple message for all countries: test, test, test."
"It has become apparent that the COVID-19 virus is highly transmissible, and a significant part of the population are asymptomatic, individuals who are currently infected or may have overcome the virus without knowing it. The immune response of these individuals can be measured by the presence of antibodies directed against proteins of the COVID-19 virus. Innovita's results are exceptionally high (specificity - true negative) at 99.57% and (sensitivity - true positive) at 86.43%. It is now accepted that widespread COVID-19 testing, identification of individuals exposed to the virus and isolation of virus-infected individuals are an effective means to control the spread of COVID-19," noted James Saunders, CEO to Emergence Technology Pty Ltd.
"This is a significant opportunity for Blackhawk" states Frederick Pels, CEO of Blackhawk Growth Corp. "This test is truly revolutionary given the fact that through a simple pin-prick of a finger supervised by a medical professional, in less than 15 minutes, it can test whether a patient has COVID-19 antibodies or not. We recognize that a number of healthcare organizations are struggling to evaluate patients for COVID-19 due to testing constraints so through this agreement we're hoping to provide an efficient solution in distributing these tests to people in Canada and parts of Europe and ease the burden on healthcare centers, so that they can focus on the highest severity cases. Giving people the ability to get tested could have immediate implications for not just the patient, but their family and friends, too and it is our mission to do everything we can to help fight this pandemic."
In consideration for the ongoing rights to distribute the Test Kits in the Acquisition Territories, the Letter contemplates that the Corporation would issue to the Vendor 20,000,000 common shares, at a deemed price of $0.05 per share, and 10,000,000 share purchase warrants entitling the Vendor to acquire additional common shares of the Corporation at a price of $0.06 per share for a period of twenty-four months. The Corporation will also grant the Vendor an ongoing royalty equivalent to nine percent of the gross revenue generated from the sale of the Test Kits in the Acquisition Territories. Completion of the transaction remains subject to completion of due diligence, the negotiation of definitive documentation, the Vendor obtaining the consent of Innovita, and completion of customary regulatory filings associated with transactions of this nature.
The Corporation is at arm's length from the Vendor and Innovita. The transaction neither constitutes a fundamental change or change of business for the Corporation, nor is it expected to result in a change of control of the Corporation within the meaning of applicable securities laws and the policies of the Canadian Securities Exchange. All securities of the Corporation issued to the Vendor will be subject to a four-month-and-one-day statutory hold period in accordance with applicable securities laws.
Readers are cautioned that the Corporation has not yet had an opportunity to conduct independent due diligence regarding the operation of the Test Kit and verification of testing statistics provided by the Vendor. At this time, use of the Test Kit has not been approved in Canada, and there can be no guarantee that such approval will be granted in a timely fashion, or at all. Assuming such approvals are received, following completion of the transaction with the Vendor, it is anticipated that the Corporation would place orders with Innovita for the manufacturing of the Test Kits in China. The Corporation has not received any assurances as to the timeline for the manufacturing and distribution of Test Kits in the Acquisition Territories, or to the capacity of Innovita to produce a sufficient volume of Test Kits to make distribution in the Acquisition Territories economically feasible.
The Corporation intends to establish this additional information through a due diligence review permitted under the terms of the Letter. The Corporation will provide a further update regarding the transaction once due diligence has been completed and definitive documentation finalized.
For further information please contact:
Frederick Pels, Chief Executive Officer
(403)-991-7737
Cautionary Note Regarding Forward-Looking Statement
All statements in this press release, other than statements of historical fact, are "forward-looking information" with respect to the Company within the meaning of applicable securities laws, including with respect to completion of the acquisition of the distribution rights for the Test Kit in the Acquisition Territories, the approval of the Test Kit by applicable local health authorities and the marketing and distribution of the Test Kit in the Acquisition Territories. The Company provides forward-looking statements for the purpose of conveying information about current expectations and plans relating to the future and readers are cautioned that such statements may not be appropriate for other purposes. By its nature, this information is subject to inherent risks and uncertainties that may be general or specific and which give rise to the possibility that expectations, forecasts, predictions, projections or conclusions will not prove to be accurate, that assumptions may not be correct and that objectives, strategic goals and priorities will not be achieved. These risks and uncertainties include but are not limited those identified and reported in the Company's public filings under the Company's SEDAR profile at www.sedar.com. Although the Company has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in forward-looking information, there may be other factors that cause actions, events or results not to be as anticipated, estimated or intended. There can be no assurance that such information will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. The Company disclaims any intention or obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise unless required by law.
Copyright (c) 2020 TheNewswire - All rights reserved.