SAN DIEGO - February 19, 2019 - (Newswire.com)
Doctors at Zaragoza University Hospitals in Spain successfully utilized microwave energy to minimally invasively treat extrahepatic bile duct tumor obstruction.
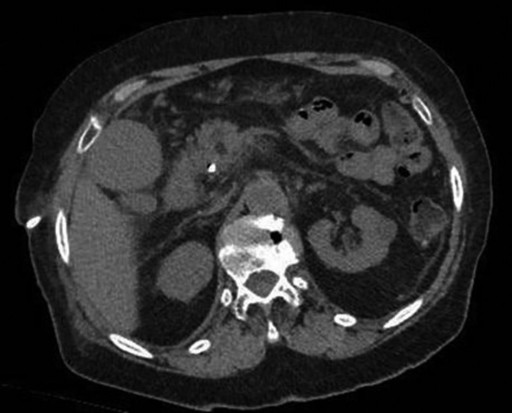
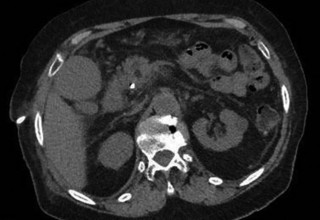
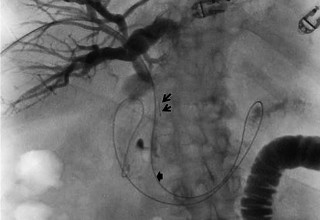
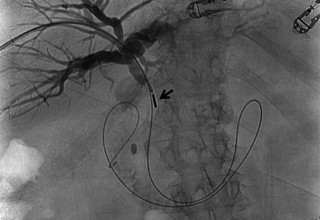
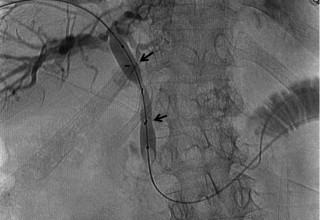
The doctors at Zaragoza University Hospital in Spain applied microwave energy to destroy tumor growth within the main bile duct to reestablish and maintain normal bile flow. A flexible catheter with a microwave antenna tip is inserted into the liver under fluoroscopy into the bile duct with blockages. Once the antenna is positioned in the blockage, microwave energy is applied using preset power, temperature and time durations endocavitary. The antenna is pulled along within the duct with the blockage, and the energy application is repeated until the desired length of the duct is treated. The doctors removed the microwave catheter and verify bile-duct flow with fluoroscopy. Doctors place resorbable prosthesis, dilation stent balloon, and 5F catheter for 24hrs. No complications were observed. The microwave energy application is controlled with direct temperature feedback from the ablation antenna during the procedure to ensure safety and efficacy.
AveCure® microwave ablation system is successfully treating tumors in bile-duct using a minimally invasive technique, the percutaneous transhepatic cholangiography (PTC) incision through the skin to access the tumor through liver, and patient is left with a small hole in the skin which quickly heals with almost no scar after the procedure. AveCure® system utilizes a smart antenna in either probe or catheter format and microwave energy controller to select the correct size, temperature, and timer settings appropriate for safe, effective and predictable treatment.
AveCure® microwave ablation system is FDA 510(K), CE Mark and COFFEPRIS registered. The MedWaves AveCure® Ablation System is intended for use in percutaneous, laparoscopic, and intraoperative coagulation-ablation of soft tissue.
The MedWaves Ablation System is not intended for use in cardiac procedures.
MedWaves, Inc is a privately held company with headquarter and manufacturing in San Diego, CA. www.medwaves.com, Tel: +1 760-807-1000, tedormsby@avecure.com
Related Images
Press Release Service by Newswire.com
Original Source: MedWaves AveCure® Ablation System: Doctors in Spain Used Microwave Energy to Successfully Treat Bile Duct Blockage